In the frame of a collaborative project co-financed by the European Union and Greek national funds (BreastCaRANKL project code: T1EDK-02829), Biomedcode has collaborated with Dr. Douni’s lab at BSRC Al. Fleming and the companies Bioemtech and Protavio to develop an innovative human-RANKL dependent breast cancer mouse model aiming to study RANKL dependent breast cancer mechanisms and to establish novel integrated preclinical platforms with modules of advanced imaging and molecular analysis for the evaluation of human therapeutics targeting cancer.
Published in Cancers 2023, 15(15), 4006.
Α Humanized RANKL Transgenic Mouse Model of Progestin-Induced Mammary Carcinogenesis for Evaluation of Novel Therapeutics
Kolokotroni A1,2, Gkikopoulou E1,2, Rinotas V2, Ntari L3, Zareifi D4, Rouchota M5, Sarpaki S5, Lymperopoulos I6, Alexopoulos LG4, Loudos G5, Denis MC3, Karagianni N3, Douni E1,2
1 Laboratory of Genetics, Department of Biotechnology, Agricultural University of Athens, Greece.
2 Institute for Bioinnovation, Biomedical Sciences Research Center “Alexander Fleming”, Vari, Greece.
3 Biomedcode Hellas SA, Vari, Greece.
4 Department of Mechanical Engineering, National Technical University of Athens, Greece.
5 BIOEMTECH, Ag. Paraskevi, Greece.
6 1st Breast Clinic, Iaso Hospital, Marousi, Greece.
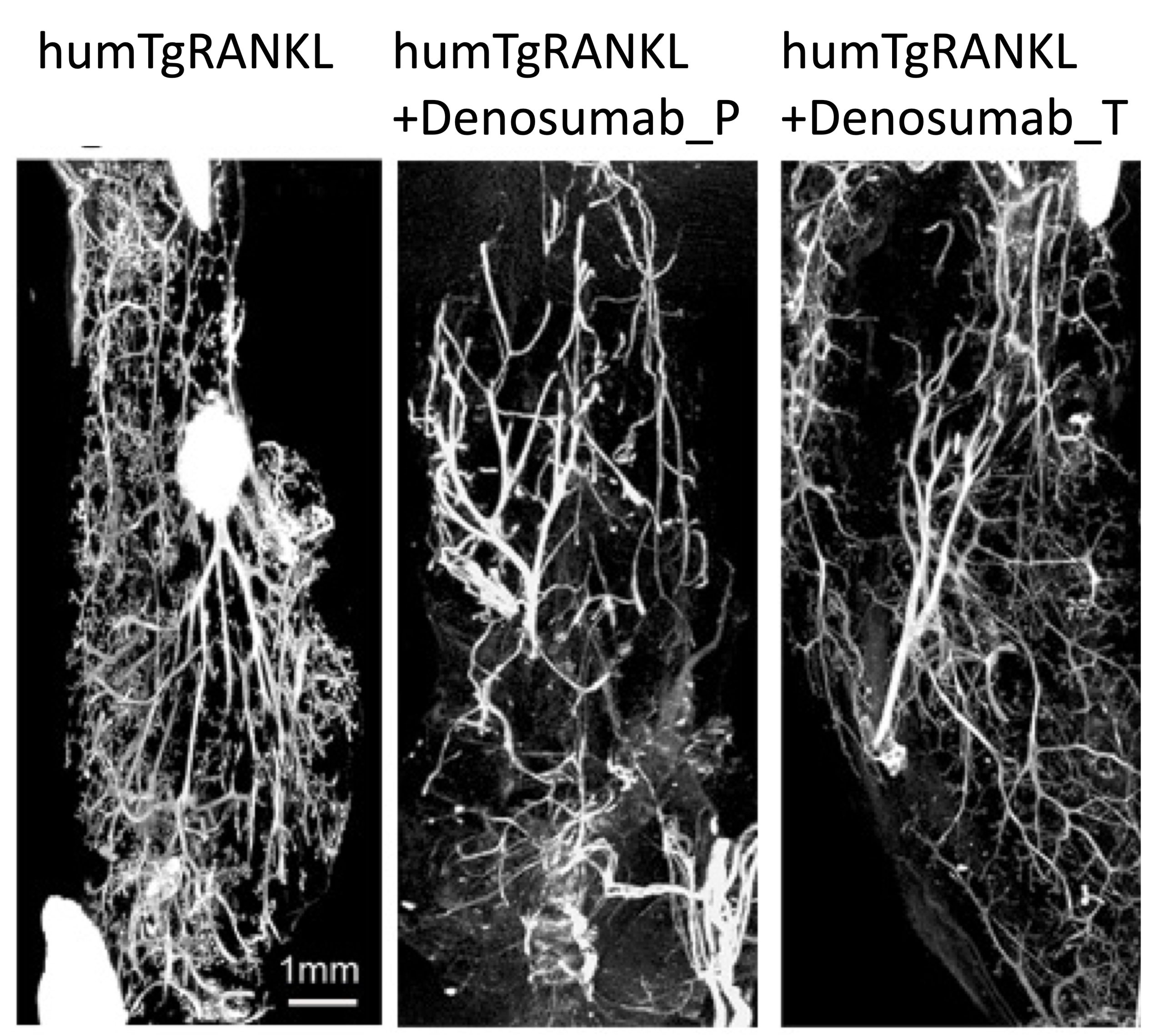
Receptor activator of nuclear factor-κB ligand (RANKL) is critically involved in mammary gland pathophysiology, while its pharmaceutical inhibition is being currently investigated in breast cancer. Herein, we investigated whether the overexpression of human RANKL in transgenic mice affects hormone-induced mammary carcinogenesis, and evaluated the efficacy of anti-RANKL treatments, such as OPG-Fc targeting both human and mouse RANKL or Denosumab against human RANKL. We established novel MPA/DMBA-driven mammary carcinogenesis models in TgRANKL mice that express both human and mouse RANKL, as well as in humanized humTgRANKL mice expressing only human RANKL, and compared them to MPA/DMBA-treated wild-type (WT) mice. Our results show that TgRANKL and WT mice have similar levels of susceptibility to mammary carcinogenesis, while OPG-Fc treatment restored mammary ductal density, and prevented ductal branching and the formation of neoplastic foci in both genotypes. humTgRANKL mice also developed MPA/DMBA-induced tumors with similar incidence and burden to those of WT and TgRANKL mice. The prophylactic treatment of humTgRANKL mice with Denosumab significantly prevented the rate of appearance of mammary tumors from 86.7% to 15.4% and the early stages of carcinogenesis, whereas therapeutic treatment did not lead to any significant attenuation of tumor incidence or tumor burden compared to control mice, suggesting the importance of RANKL primarily in the initial stages of tumorigenesis. Overall, we provide unique genetic tools for investigating the involvement of RANKL in breast carcinogenesis, and allow the preclinical evaluation of novel therapeutics that target hormone-related breast cancers.