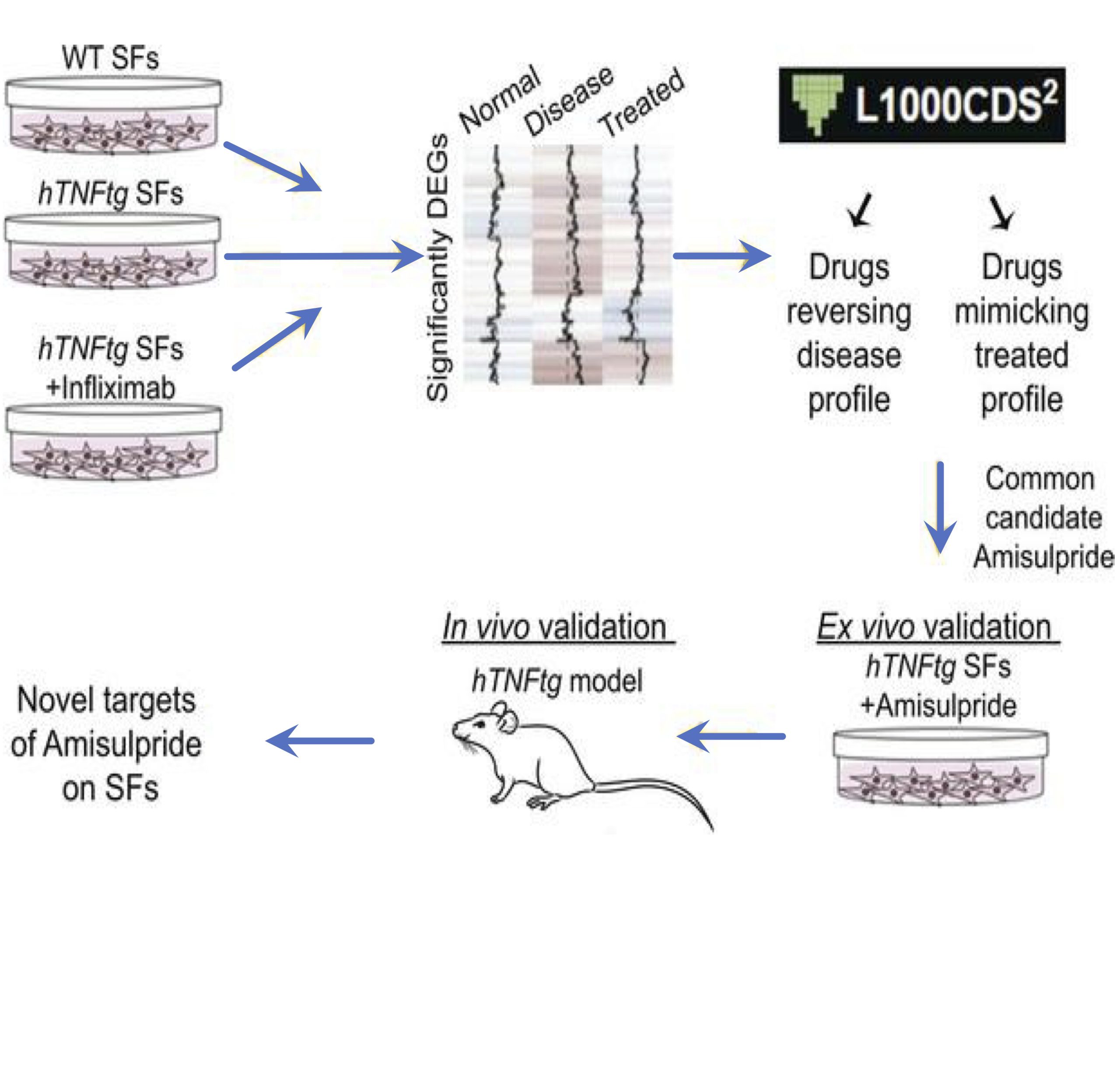
In the frame of a collaborative drug development project scientists from Biomedcode and BSRC Al. Fleming using bioinformatics tools, have repurposed the neuroleptic drug amisulpride for the reversal of the pathogenic expression signature of synovial fibroblasts and the treatment of arthritis pathology.
Published in JCI Insight 2023 May 8;8(9):e165024. doi: 10.1172/jci.insight.165024.
Dimitra Papadopoulou,1 Fani Roumelioti,1 Christos Tzaferis,1,2 Panagiotis Chouvardas,1 Anna-Kathrine Pedersen,3 Filippos Charalampous,1 Eleni Christodoulou-Vafeiadou,4 Lydia Ntari,4 Niki Karagianni,4 Maria C. Denis,4 Jesper V. Olsen,3 Alexios N. Matralis,1 and George Kollias1,2,5
1Institute for Bioinnovation, Biomedical Sciences Research Centre Alexander Fleming”, Vari, Greece. 2Department
of Physiology, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece. 3Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark. 4Biomedcode Hellas SA, Vari, Greece. 5Center of New Biotechnologies & Precision Medicine, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Synovial fibroblasts (SFs) are key pathogenic drivers in rheumatoid arthritis (RA). Their in vivo activation by TNF is sufficient to orchestrate full arthritic pathogenesis in animal models, and TNF blockade proved efficacious for a high percentage of patients with RA albeit coinducing rare but serious side effects. Aiming to find new potent therapeutics, we applied the L1000CDS2 search engine, to repurpose drugs that could reverse the pathogenic expression signature of arthritogenic human TNF–transgenic (hTNFtg) SFs. We identified a neuroleptic drug, namely amisulpride, which reduced SFs’ inflammatory potential while decreasing the clinical score of hTNFtg polyarthritis. Notably, we found that amisulpride function was neither through its known targets dopamine receptors D2 and D3 and serotonin receptor 7 nor through TNF–TNF receptor I binding inhibition. Through a click chemistry approach, potentially novel targets of amisulpride were identified, which were further validated to repress hTNFtg SFs’ inflammatory potential ex vivo (Ascc3 and Sec62), while phosphoproteomics analysis revealed that treatment altered important fibroblast activation pathways, such as adhesion. Thus, amisulpride could prove beneficial to patients experiencing RA and the often-accompanying comorbid dysthymia, reducing SF pathogenicity along with its antidepressive activity, serving further as a “lead” compound for the development of novel therapeutics against fibroblast activation.