The Disease
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system that leads to paralysis and currently affects approximately 2.5 Million people world wide. The exact cause of MS is unknown and the disease is non-curable.
The rising in disease prevalence and the technological advances are attracting the interest of pharmaceutical companies aiming at developing therapeutics targeting MS.
Our Preclinical Testing Tools
Biomedcode has standardized preclinical evaluation platforms based on the MOG-induced Experimental Autoimmune Encephalomyelitis (EAE) the most widely used animal model of multiple sclerosis. This model combined with Biomedcode’s humanized mice allows the direct assessment of the therapeutic efficacy of human therapeutics targeting TNFR1 and IL17.
Drug Efficacy Evaluation Platforms
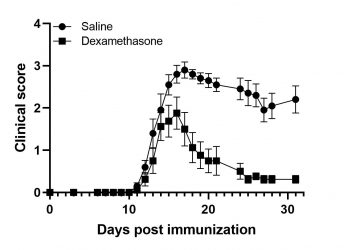
EAE is induced following the immunization of animals with MOG 35-55 peptide in complete Freund’s adjuvant and the concomitant administration of Pertussis Toxin. The symptoms of the disease manifest with progressive paralysis with the first signs appearing 10 days after the initial immunization. The severity of the signs is assessed regularly using a standardized in vivo scoring scale. Treatment with corticosteroids, anti-hIL17 or anti-hTNFR1 reverse symptoms.
Read-Out Parameters
In vivo EAE scoring
Body weight measurements
Histology (qualitative observations)
Platform validation
MOG-induced EAE in Wild-Type mice
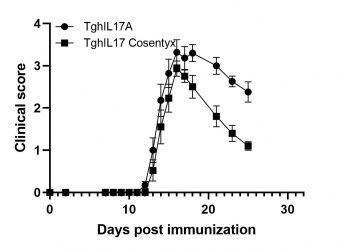
MOG-induced EAE in TghIL17 mice
Biomedcode’s unique IL17 humanized mice express exclusively human IL17. These mice have normal phenotype and in combination to EAE induction protocols they offer a unique tool for the evaluation of the efficacy of anti-human IL17 therapeutics in the treatment of multiple sclerosis.
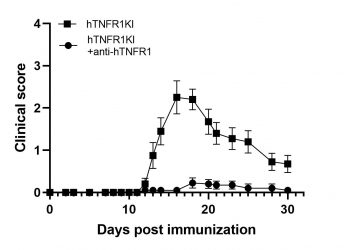
MOG-induced EAE in hTNFR1KI mice
The huTNFR1KI mice are humanized for TNFR1 and in combination with the EAE induction protocol they offer a unique tool for the evaluation of the efficacy of anti-human TNFR1 therapeutics targeting the treatment of multiple sclerosis.
Competitive Advantage
MOG induced EAE is an established animal model of multiple sclerosis reproducing the majority of the pathology features of human condition. Standardization and validation of the model by our experienced scientific personnel has provided reliable preclinical evaluation platforms for the efficacy assessment of candidate multiple sclerosis therapeutics. Induced EAE using humanized IL17 mice (TghIL17) or hTNFR1KI mice allows the evaluation of biologics targeting IL17 or TNFR1 respectively.