With a new publication in Arthritis Research and Therapy, entitled “Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis”, Biomedcode in collaboration with George Kollias Lab at BSRC Al. Fleming, introduce the TgA86 transmembrane TNF transgenic mouse as a novel model of human spondyloarthritis (SpA).
The authors show that the TgA86 mouse model develops spontaneously peripheral arthritis and axial pathologies that closely reproduce key pathogenic features of human SpA, including distinct stages of inflammation and ectopic new bone formation. This is a chronic and complex disease model that similar to human patients also develops extraarticular comorbidities such as heart valve pathology and systemic bone loss. As with human patients in the clinic, all the pathologies of the TgA86 mouse model are reversed following early treatment with anti-hTNF therapeutics.
This novel model of SpA that captures not only specific features, but also the complexity of human disease, can prove to be an invaluable translational tool in the study of SpA pathogenesis as well as in the evaluation of human therapeutics.
Published in Arthritis Research and Therapy 2020 Oct 6;22(1):232. doi: 10.1186/s13075-020-02327-4.
Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis
Christodoulou-Vafeiadou E, Geka C, Ntari L, Kranidioti K, Argyropoulou E, Meier F, Armaka M, Mourouzis I, Pantos C, Rouchota M, Loudos G, Denis MC, Karagianni N, Kollias G
Background: The transmembrane-TNF transgenic mouse, TgA86, has been shown to develop spontaneously peripheral arthritis with signs of axial involvement. To assess similarity to human spondyloarthritis, we performed detailed characterization of the axial, peripheral, and comorbid pathologies of this model.
Methods: TgA86 bone pathologies were assessed at different ages using CT imaging of the spine, tail vertebrae, and hind limbs and characterized in detail by histopathological and immunohistochemical analysis. Cardiac function was examined by echocardiography and electrocardiography and bone structural parameters by μCT analysis. The response of TgA86 mice to either early or late anti-TNF treatment was evaluated clinically, histopathologically, and by μCT analysis.
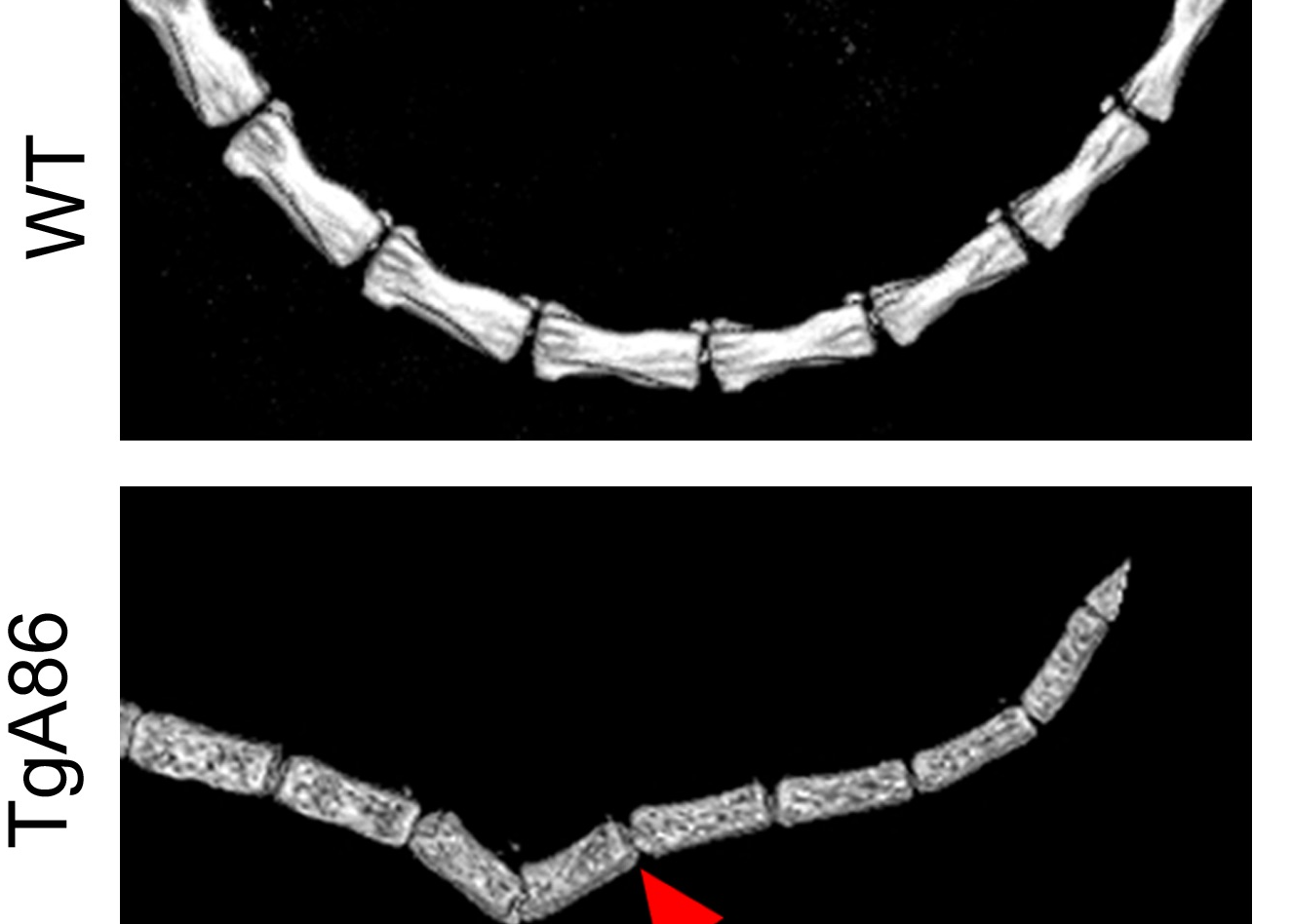
Results: TgA86 mice developed with 100% penetrance spontaneous axial and peripheral pathology which progressed with time and manifested as reduced body weight and body length, kyphosis, tail bendings, as well as swollen and distorted hind joints. Whole-body CT analysis at advanced ages revealed bone erosions of sacral and caudal vertebrae as well as of sacroiliac joints and hind limbs and, also, new ectopic bone formation and eventually vertebral fusion. The pathology of these mice highly resembled that of SpA patients, as it evolved through an early inflammatory phase, evident as enthesitis and synovitis in the affected joints, characterized by mesenchymal cell accumulation, and neutrophilic infiltration. Subsequently, regression of inflammation was accompanied by ectopic bone formation, leading to ankylosis. In addition, both systemic bone loss and comorbid heart valve pathology were evident. Importantly, early anti-TNF treatment, similar to clinical treatment protocols, significantly reduced the inflammatory phase of both the axial and peripheral pathology of TgA86 mice.
Conclusions: The TgA86 mice develop a spontaneous peripheral and axial biphasic pathology accompanied by comorbid heart valvular dysfunction and osteoporosis, overall reproducing the progression of pathognomonic features of human spondyloarthritis. Therefore, the TgA86 mouse represents a valuable model for deciphering the role of transmembrane TNF in the pathogenic mechanisms of spondyloarthritis and for assessing the efficacy of human therapeutics targeting different phases of the disease.