Tg3647 mice over-express human TNF that leads to the gradual development of spontaneous slow progressing chronic inflammatory polyarthritis with 100% penetrance.


Tg3647 mice over-express human TNF that leads to the gradual development of spontaneous slow progressing chronic inflammatory polyarthritis with 100% penetrance.

Targeting TNF-α as a treatment modality has shown tremendous success, however there are several limitations associated with the current anti-TNF-α biologic drugs including: immunogenicity, life-threatening infections, resistance to treatment, complexity of manufacture and cost of treatment. Ubah et al. report the in vivo efficacy of novel anti-TNF-α formats generated from molecular engineering of variable new antigen receptors (VNARs), originally derived from the immune system of an immunized nurse shark.
Published in Frontiers in Immunology 2019 Mar 22;10:526. doi: 10.3389/fimmu.2019.00526.
Collagen antibody-induced arthritis (CAIA) is a simple mouse model of rheumatoid arthritis that can be used to address questions relating to the pathogenic mechanisms of the disease and serves as a platform for the evaluation of candidate therapeutic agents.
Biomedcode has standardized the model in
and developed platforms to allow the evaluation of different arthritis therapeutics.
Scientists of Biomedcode present in PLOS Computational Biology a new computational framework revealing key differences between four rheumatoid arthritis medications and their impact on biological pathways in mice.
Published in PLoS Computational Biology 2019 May 9;15(5):e1006933. doi: 10.1371/journal.pcbi.1006933
Tg54531 is a transgenic mouse with transmembrane human TNF deregulated expression resulting in the spontaneous development of arthritis pathology that closely resembles human rheumatoid arthritis.
The mice develop arthritis with 100% penetrance and provide a fast in-vivo model for evaluating human therapeutics targeting rheumatoid arthritis.
The Tg5453 mouse model was successfully used in establishing the therapeutic efficacy of Remicade®, the first anti-TNF therapeutic to be successfully applied in the clinic, and is currently used for screening anti-rheumatoid candidate drugs.

The TNFΔARE mouse model develops arthritis pathology together with Crohn’s like ileal inflammation with 100% penetrance. The co-occurence of two inflammatory pathologies provide a reliable in-vivo model for evaluating therapeutics targeting both pathologies. Read More
The Tg197 mouse model was successfully used in establishing the therapeutic efficacy of Remicade®, the first anti-TNF therapeutic to be successfully applied in the clinic, and is recommended by the FDA for screening potential anti-rheumatoid candidate drugs.
Tg197 is a transgenic mouse overexpressing human TNF resulting in the spontaneous development of arthritis whose pathology closely resembles human rheumatoid arthritis. The mice develop arthritis with 100% penetrance and provide a fast in-vivo model for evaluating human therapeutics targeting rheumatoid arthritis.
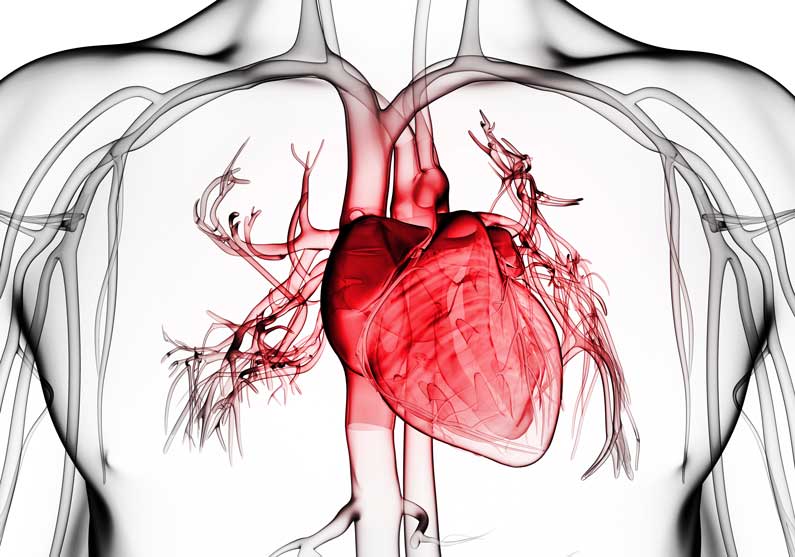
Patients with rheumatoid arthritis and spondyloarthritis show higher mortality rates, mainly caused by cardiac comorbidities. The TghuTNF (Tg197) arthritis model develops tumour necrosis factor (TNF)-driven and mesenchymal synovial fibroblast (SF)-dependent polyarthritis. Here, we investigate whether this model develops, similarly to human patients, comorbid heart pathology and explore cellular and molecular mechanisms linking arthritis to cardiac comorbidities.
Published in Annals of Rheumatic Diseases 2018 Jun;77(6):926-934. doi: 10.1136/annrheumdis-2017-212597.

Biomedcode scientists coauthor two publications, one in Annals of Rheumatic diseases and one in JCI insight, showing that Tg197 and TNFΔΑRΕ mice develop heart valve pathology sharing common mesenchymal cell-specific aetiopathogenic mechanisms with arthritis. Read More

Mesenchymal TNF signaling is etiopathogenic for inflammatory diseases such as rheumatoid arthritis and spondyloarthritis (SpA). The role of Tnfr1 in arthritis has been documented; however, Tnfr2 functions are unknown. Here, we investigate the mesenchymal-specific role of Tnfr2 in the TnfΔARE mouse model of SpA in arthritis and heart valve stenosis comorbidity by cell-specific, Col6a1-cre-driven gene targeting. We find that TNF/Tnfr2 signaling in resident synovial fibroblasts (SFs) and valvular interstitial cells (VICs) is detrimental for both pathologies, pointing to common cellular mechanisms. In contrast, systemic Tnfr2 provides protective signaling, since its complete deletion leads to severe deterioration of both pathologies. SFs and VICs lacking Tnfr2 fail to acquire pathogenic activated phenotypes and display increased expression of antiinflammatory cytokines associated with decreased Akt signaling.
Published in JCI Insight 2018 Apr 5;3(7):e98864. doi: 10.1172/jci.insight.98864
Read More