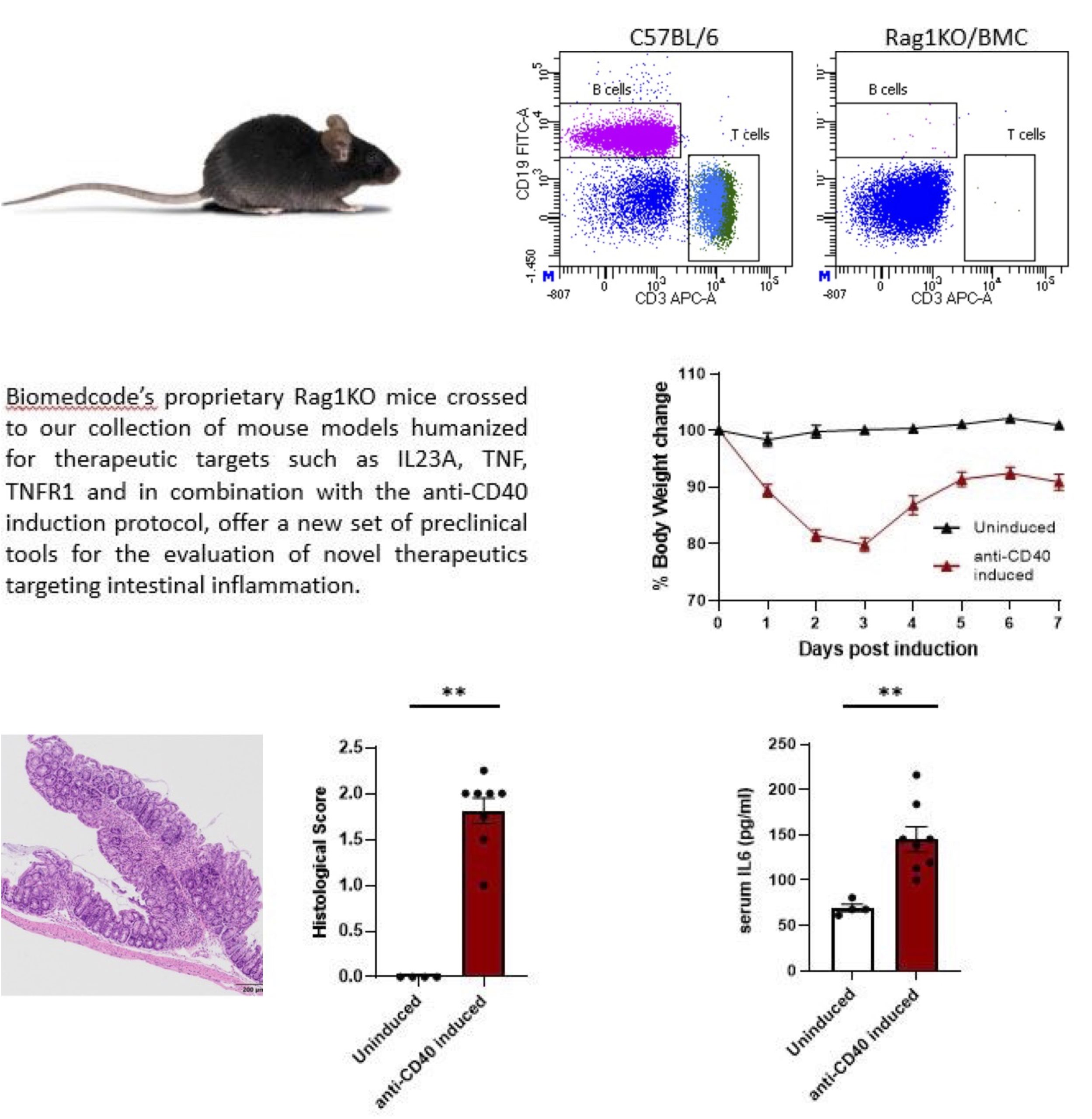
We are excited to introduce a new addition to our collection of genetically modified mice used in human disease modelling. We have now developed and characterized a proprietary Rag1KO/BMC mouse line that either alone or in combination with our collection of proprietary mouse lines allows us to offer a new series of anti-CD40 colitis mouse models that can serve as invaluable preclinical tools to better understand and treat the pathogenic mechanisms involved in the development and progression of Inflammatory Bowel Disease (IBD).
💉 The anti-CD40 model of colitis is a valuable model to study innate immune responses in colon inflammation and to evaluate the therapeutic effect of TNF, IFNγ or IL-12/IL-23 p40 inhibition 💊
What is the anti-CD40 induced colitis model?
Activation of CD40 by an agonistic anti-CD40 antibody in mice lacking T and B cells, as the Rag1 or Rag2 knockout mice, leads to the activation of innate immune responses, the excessive production of IL-23, IL-1β and IL-12 and the development of inflammation in the colon.
The anti-CD40 induced colitis pathology is characterized by body weight loss, increased levels of circulating cytokines, reduced colon length and increased colon weight, accompanied by relevant histopathological signs of gut inflammation and extensive crypt destruction.